Real-time monitoring of solid-phase peptide synthesis
The production of peptides is now an indispensable part of modern pharmaceutical research and production. Peptides are organic compounds consisting of amino acids linked by peptide bonds. They are one of the most important classes of active pharmaceutical ingredients (APIs) and form the basis of numerous drugs. They are used in cancer, diabetes, and autoimmune therapies, among others. At the same time, however, their production is complex, time-consuming, and involves high solvent consumption. Solid-phase peptide synthesis (SPPS) is a commonly used method for producing peptides.
Process of solid-phase peptide synthesis
Solid-phase peptide synthesis was developed in 1963 by Bruce Merrifield and revolutionized peptide chemistry. Its principle is based on the growing peptide chain being bound to an insoluble resin suspended in a solvent.
The synthesis process consists of recurring cycles of:
-
-
- Coupling: A protected amino acid is added to the free binding end of the peptide chain together with coupling reagents.
- Washing: Unreacted amino acids and excess reagents are removed by rinsing.
- Removal of the protective group: The protective group of the newly added amino acid is removed to expose the next binding end.
- Repeated washing: Residues of the protective groups and by-products are removed.
This cycle is repeated until the desired sequence is completely built up. Finally, the entire peptide chain is cleaved from the resin and purified.
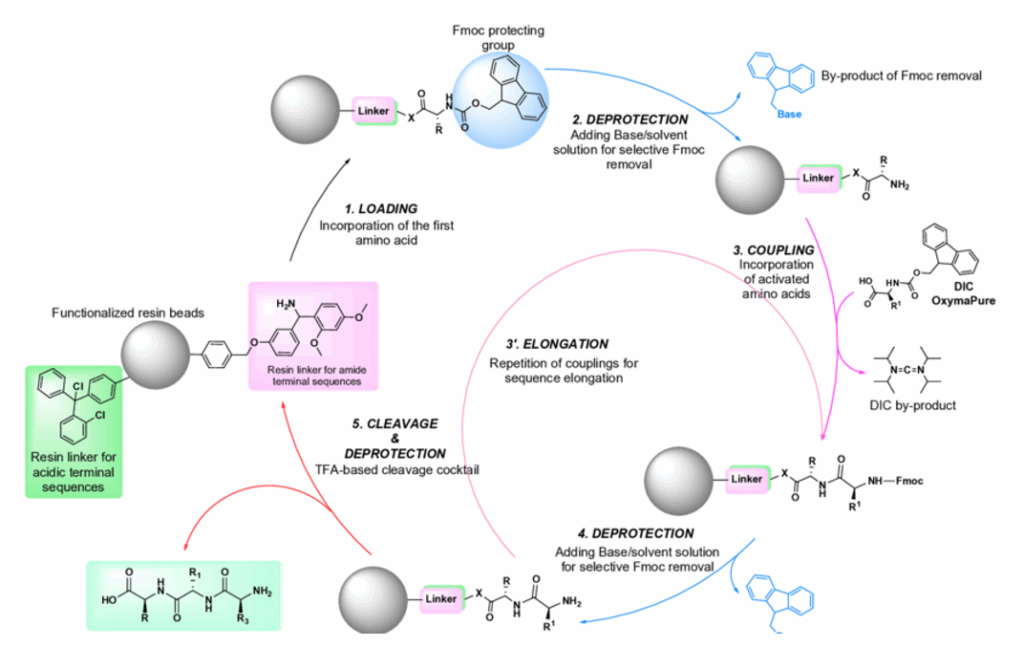 Figure 1: Example of an SPPS process (Source: https://www.researchgate.net/figure/General-scheme-of-solid-phase-peptide-synthesis-SPPS-steps_fig4_366806988)
Challenges in peptide synthesis
Figure 1: Example of an SPPS process (Source: https://www.researchgate.net/figure/General-scheme-of-solid-phase-peptide-synthesis-SPPS-steps_fig4_366806988)
Challenges in peptide synthesis
Continuous process monitoring is therefore a decisive factor for the quality and cost-effectiveness of solid-phase peptide synthesis. Previous methods such as HPLC analysis, ninhydrin or chloranil tests, and UV absorption can provide valuable information, but they have serious disadvantages: They are time-consuming, destructive, require sampling, and often lead to interruptions in the entire production process. SPPS is like a black box, as no intermediate products can be isolated during the ongoing process and the end result remains uncertain until the synthesis is complete. This is where inline or online measurement with a refractometer comes in. The refractive index (RI) of a solution depends directly on its composition. Every reaction—whether it is the binding of an amino acid to the resin, the removal of a protective group, or a washing step—changes the concentration of the dissolved substances in the system and thus the refractive index. These changes can be measured precisely in real time with modern refractometers. This makes solid-phase peptide synthesis transparent and controllable.
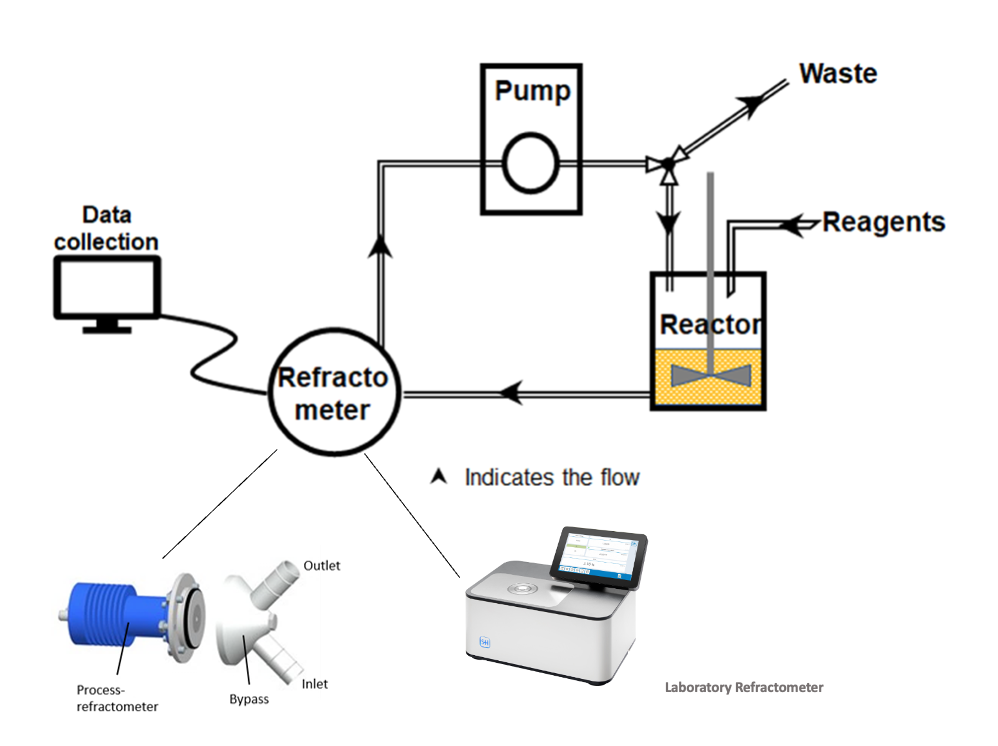 Figure 2: Schematic diagram of an SPPS with refractometers (Source: https://pubs.acs.org/doi/10.1021/acs.oprd.1c00051)
Figure 2: Schematic diagram of an SPPS with refractometers (Source: https://pubs.acs.org/doi/10.1021/acs.oprd.1c00051)
A refractometer offers the great advantage of being a non-destructive and continuous measurement method that does not require sampling. The data is available immediately and provides valuable information about the progress of each individual synthesis step. For example, the loading of the resin can be tracked live. Capping steps or the removal of protective groups can also be identified by characteristic signal curves. Washing cycles with solvents such as DMF are also clearly visible in the measurements and can thus be optimized in terms of efficiency and duration.
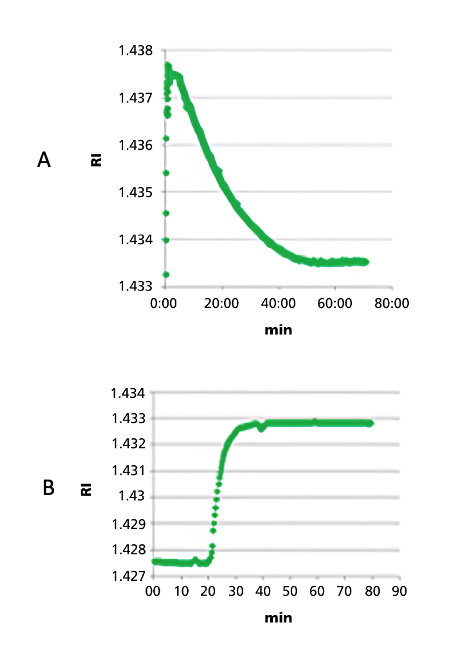 Figure 4: RI curve during the coupling process (A) and removal of the protective group (B) (Source: https://pubs.acs.org/doi/10.1021/acs.oprd.1c00051)
Figure 4: RI curve during the coupling process (A) and removal of the protective group (B) (Source: https://pubs.acs.org/doi/10.1021/acs.oprd.1c00051)
This not only increases process reliability, but also efficiency: production times are reduced from several hours in some cases to a few minutes, reagents can be used more sparingly, and solvent consumption is reduced. In this way, a refractometer contributes significantly to more sustainable and economical peptide production – in line with modern “green chemistry” approaches. In addition, real-time monitoring enables extensive automation of SPPS and creates the basis for reliable scaling from laboratory trials to industrial production.
To make the most of these advantages, SCHMIDT + HAENSCH offers solutions for various areas of application: The VariRef laboratory refractometer, for example, is suitable for smaller laboratory systems. In combination with the flow cell door and a diaphragm pump, volumes from a few milliliters to several liters can be monitored without any problems. For industrial scale, the iPR B4 inline process refractometer can be used, for example. This inline refractometer can be installed in a bypass system as well as directly inline in reactors or pipelines. It is designed for continuous operation and allows continuous process control even under demanding production conditions.
Your advantages at a glance
-
- Significant time savings in all steps
- Real-time data instead of time-delayed offline analyses
- Continuous, non-destructive measurement without sampling
- Precise endpoint detection during coupling and removal of the protecting group
- Optimized washing steps and reduced solvent consumption
- Greater process reliability, reproducibility, and automation
- Cost savings through reduced reagent consumption
- More sustainable synthesis through less waste and lower energy requirements
SCHMIDT + HAENSCH refractometers thus offer a comprehensive tool for process control in peptide synthesis. They combine maximum precision with user-friendliness and enable a significant increase in quality while saving time and money. These technologies make peptide synthesis not only more efficient but also more sustainable—from basic research to large-scale production.


