Educational
What is refractometry?
Using optical principles to analyze substances
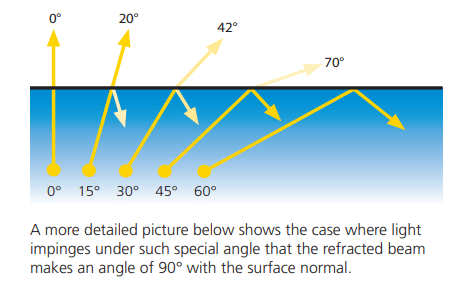
Refractometry is a quantitative, non-destructive measuring technique based on optical principles that are evident in nature: For example, when light passes through water, the light beam is reflected – and objects under water appear to have different proportions than those observed above water. This phenomenon is called reflection and refraction.
Refraction and reflection of light occur when light hits the interface of a media with different refractive indices. Therefore, refractometry is generally the measurement of refractive index and its interpretation under different initial conditions. Refractometric measurements can be performed in laboratories or in-line, meaning during a production process of liquid substances of any kind.
The examination and quality control of oils, fats, sugar, and sugar- containing substances are the traditional main areas of refractive measurement. Additionally, lemonade, beer and alcoholic beverages belong to the material class which are typically analyzed by a sensor.
Most liquid and translucent solid samples are measured with refractometry. To learn more about our measuring scales for refractometers, click here.
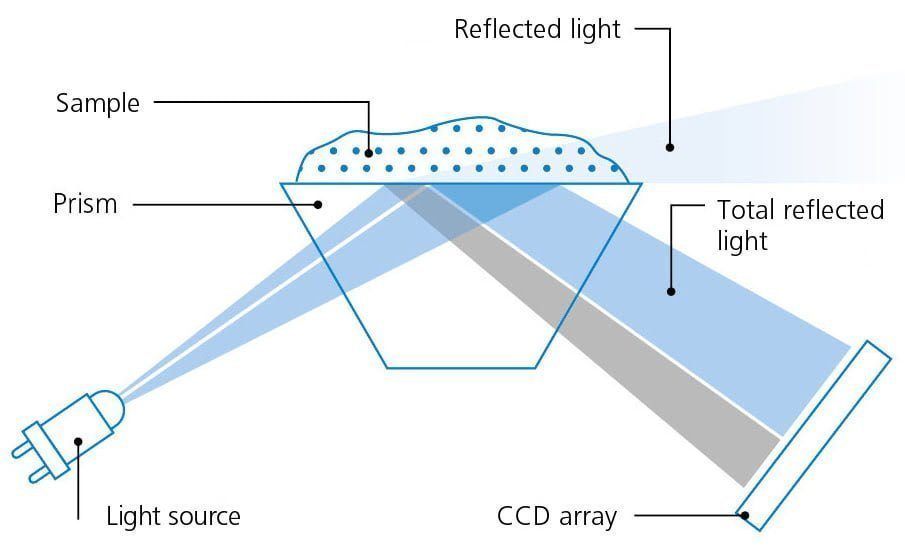
Refractometry is thus a means for controlling the quality of substances.
The refractive index is a specific value for a material. It depends on the temperature and the wavelength (λ = color) of the light. A refractometer can therefore be used to determine the concentration of a substance if the temperature and wavelength are known. It is also possible that different materials have the same refractive index at various concentrations. Thus, a clear determination of liquid substances may only be successful with binary mixtures (Mixtures consisting of two compounds).
In practice, the refractive index determines the mixing ratio quite accurately and simply, even of multi-component solutions, since usually only the concentration of one of the components needs to be determined. It is therefore a quantitative measurement. For known compounds, also quality can also be determined. For such as olive oil or orange juice, the measuring value within a certain range corresponds to a certain quality.
There is a definite correlation between the refractive index and the composition of many two-compound solutions. The best-known example of such a mixture is a solution of sucrose in water, which has been studied thoroughly. The refractive index of a refractometer can be scaled so that the value directly indicates the dry substance in % RTS. For sucrose, this unit is also named Brix (abbreviation Brix).
The density of samples can also be determined refractometrically. As a rule, density and dry substance correspond to each other in optical measurements.
Refractometers convert the measured refractive indices into concentration or density.
Refractometry and Polarimetry: Complementary Techniques
Refractometry and polarimetry often work hand in hand to deliver a comprehensive analysis of complex samples. For example, while refractometry measures the refractive index to determine concentration or density, polarimetry provides information on optical rotation, allowing precise characterization of optically active substances like essential oils, sugar solutions, or pharmaceutical compounds.
In essential oil analysis, the refractive index helps verify purity, while specific rotation—measured with a polarimeter—confirms authenticity and composition. For sugar solutions, refractometry can determine the Brix value (dry substance in % RTS), while polarimetry assesses the optical rotation to ensure compliance with product standards.
Do you have any questions?
We will be happy to help you


